
Neutral, so # = # 1 H 1 H 1.01 1 1S -1 H3- 3H- H+ 3H SHOW ME THE END PRODUCT! NOW! 2H H PTĢ3 neutral, so # = # 1 H 1 H 1.01 1 1S -1 H3- 3H- H+ 3H 2H H PT Neutral, so # = # 2 He 2 He 4.00 2 4S -2 2 SHOW ME THE END PRODUCT! NOW! He2+ He PTĢ1 neutral, so # = # 2 He 2 He 4.00 2 4S -2 2 He2+ He PT Neutral, so # = # 6 C 6 C 12.01 6 12S - 6 6 oh noes! SHOW ME THE END PRODUCT! NOW! 14C C PTĩ neutral, so # = # 6 C 6 C 12.01 6 12S - 6 6 14C C PT Neutral, so # = # 3 Li 3 Li 6.94 3 7S -3 4 SHOW ME THE END PRODUCT! NOW! Li+ Li PTĥ neutral, so # = # 3 Li 3 Li 6.94 3 7S -3 4 Li+ Li PTġ missing, so 3 – 1 = 2 Li + 3 Li 6.94 3 7S -3 4 SHOW ME THE END PRODUCT! NOW! Li+ Li PTħ 1 missing, so 3 – 1 = 2 Li + 3 Li 6.94 3 7S -3 4 Li+ Li PT
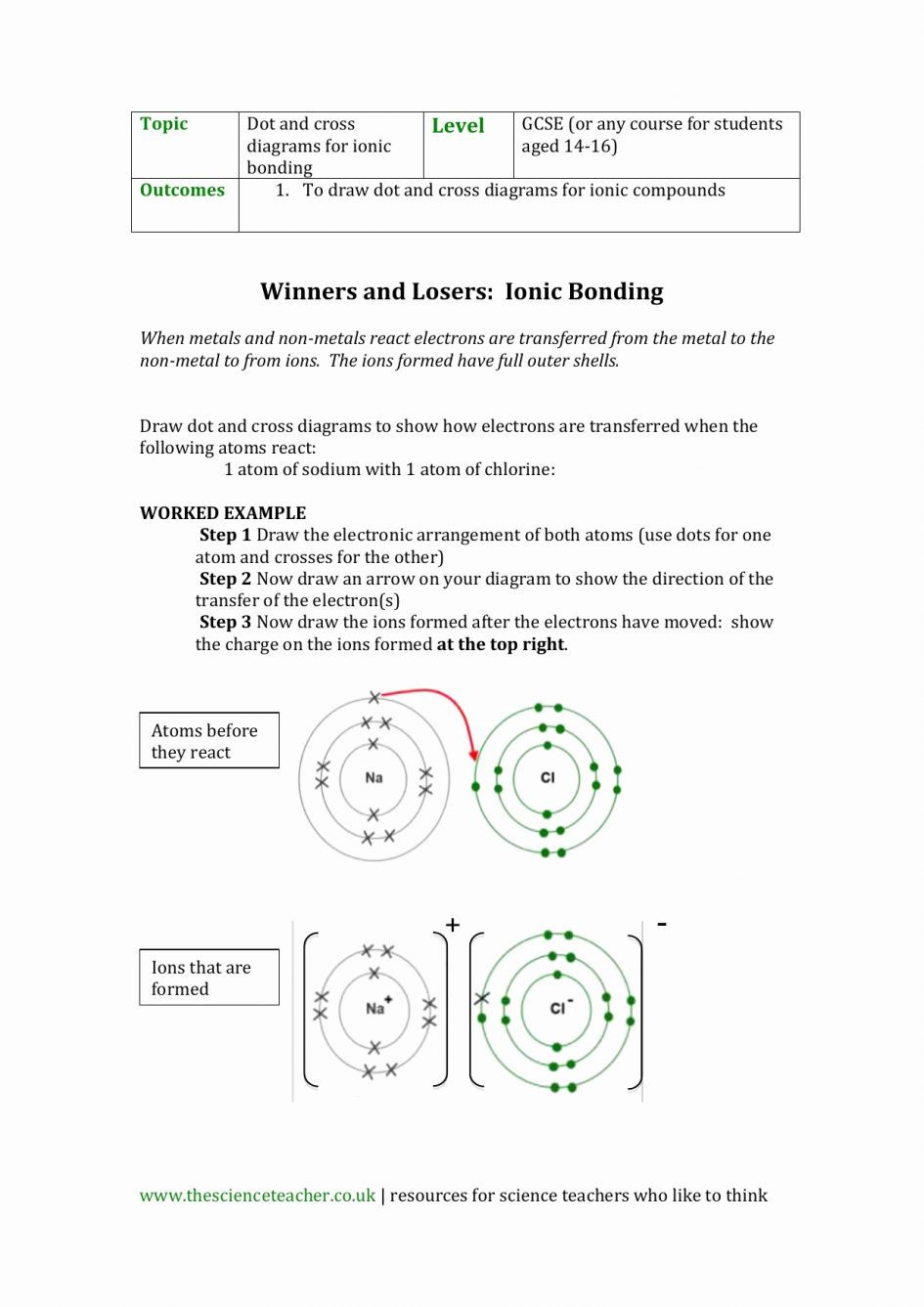
draw an atom when given a chemical symbol with a mass number and an electric charge. 1 Atom Drawing Worksheet Answer Key Daniel R.


 0 kommentar(er)
0 kommentar(er)
